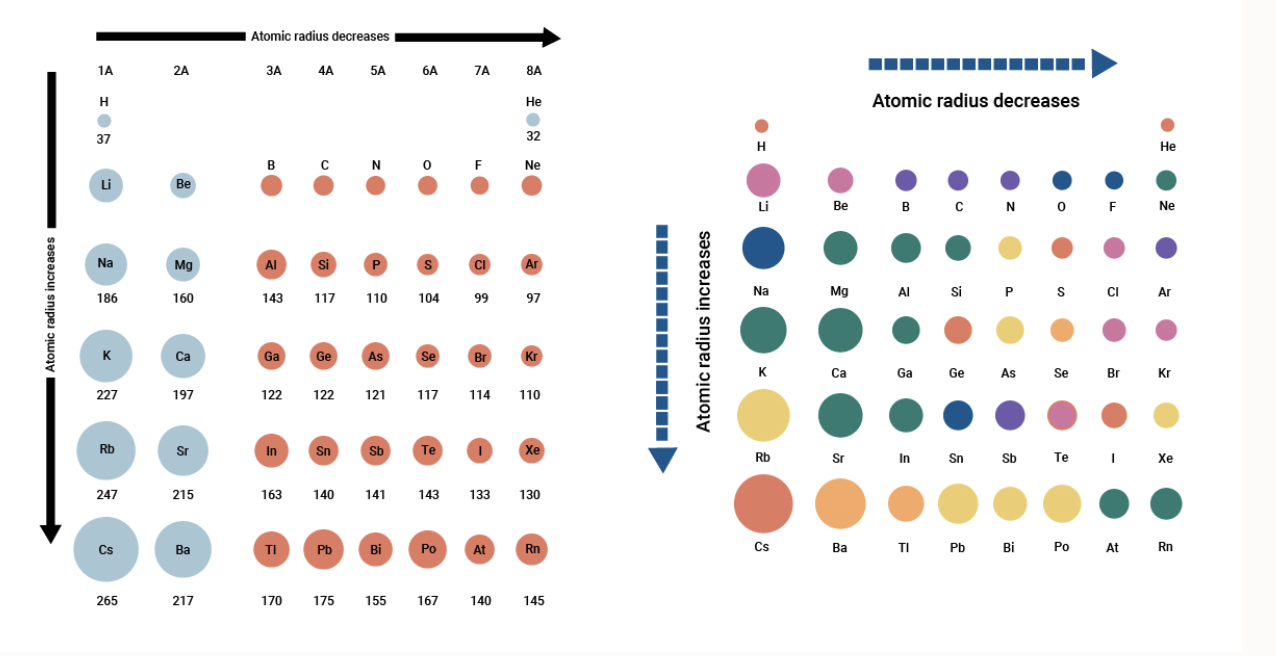
Across a period atomic radii decrease. The Ionic radii decreases then increase and then decreases.
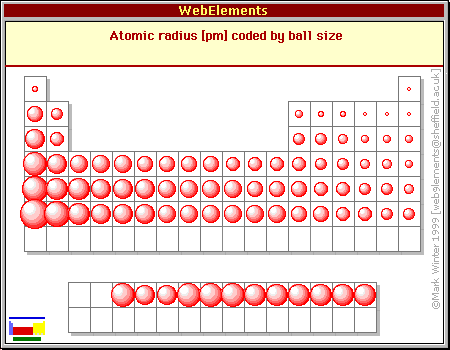
How Does Atomic Radius Change From Left To Right Across A Period In The Periodic Table Socratic
The atomic radius of an atom is the distance from the atoms nucleus to its outermost electron.

. Atomic and Ionic Radii of the Period 3 Elements. Atomic Radii Given that atoms and ions are subject to different environments and forces it is difficult to assign atomicionic radii - the electron density clouds shape and size is constantly modified 1. These topics are covered in various places elsewhere on the site and this page simply brings.
It covers ionisation energy atomic radius electronegativity electrical conductivity melting point and boiling point. Across the period the atomic radii decrease. This is because the number of protons increases sodium has 11 argon has 18 so the nuclear charge increases.
Trends in atomic radius across periods. Atoms and ions are also rarely considered in isolation. This is because while the number of electrons increases down the period they only add to the same main energy level and therefore do not.
Trends in Ionic Radius Across a Period In period 3 we find that the atomic radius first decreases and then suddenly increases and then again it slowly decreases. The atomic radius of the elements decreases from sodium to argon. Therefore the attraction between the positive nucleus and negative electrons in the outer shell increases so.
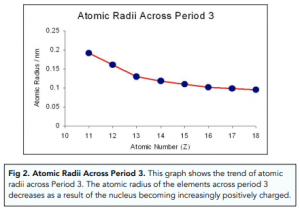
This question is about trends in the Period 2 elements from lithium to nitrogen. The graph shows a decrease in atomic radii of period 3 elements across the period. 13 rows The following general trends are observed as you go across period 3 from left to right.
You have to ignore the noble gas at the end of each period. You can see a clear trend across the period. Moving across Period 3 the number of protons in the nucleus increases - for example sodium has 11 protons and chlorine has 17 protons.
What is the trend in atomic radius of the elements across Period 3 and why does this occur. In general atomic radius decreases across a period and increases down a groupAcross a period effective nuclear charge What is the trend for atomic size from top to bottom in a group. For example ionization energy electronegativity and of course atomic radius which we will discuss now.
Up to 24 cash back Trends in physical properties occur across all Periods in the Periodic Table. Lets look at the radii of the simple ions formed by elements as you go across Period 3 of the. Why does electronegativity decrease down a group.
There are many trends on the periodic table. A Identify from the Period 2 elements lithium to nitrogen. Because neon and argon dont form bonds you can only measure their van der Waals radius - a case where the atom is pretty well unsquashed.
This is because the starting elements in a period tend to form cations and the elements. Atomic and Ionic radii of Period 3 in the periodic table The atomic radii increases because there is an increase in effective nuclear charge because of the increase of atomic numbers. Nuclear charge increases across the period therefore the attraction between the positively charged nucleus and.
This page describes and explains the trends in atomic and physical properties of the Period 3 elements from sodium to argon. This is because the number of protons the nuclear charge and the number of electrons increases by one every time you go an element to the right.
How Does Atomic Radius Change As You Move Across The Periodic Table Quora
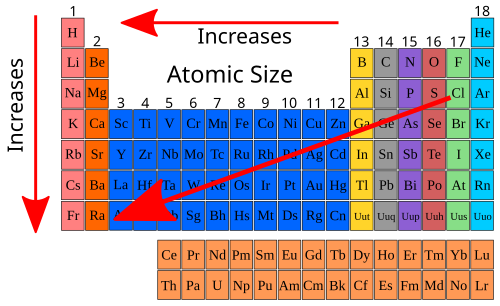
High School Chemistry Atomic Size Wikibooks Open Books For An Open World

Periodic Trends And Atomic Radius Chad S Prep

How Does Atomic Size Vary On Moving Across A Period From Left To Right And In A Group Top To Bottom Why Quora
3 2 Periodic Trends Ib Alchemy

Periodic Table Trends Atomic Size Melting Boiling Point Trend

A Level Z 1 To 20 Periodicity Plots Graphs Of Physical Properties Of Elements Of The Periodic Table Gce As A2 Inorganic Revision Notes Ks5
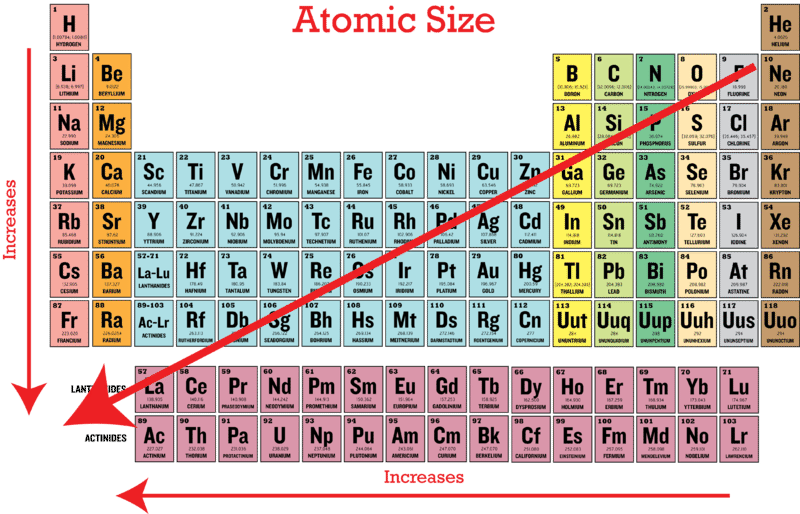
Periodic Trends In Atomic Size Ck 12 Foundation
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

6 15 Periodic Trends Atomic Radius Chemistry Libretexts

Trends In Atomic Radius And Ionic Radius Definition Examples Diagrams

Suka Chemistry Atomic Radius Trends On Periodic Table

Inorganic Chemistry Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange

A Level Gce Period 3 Element Trends In 1st Ionisation Energy Atomic Radius Pauling Electronegativity Melting Point Boiling Point Electrical Conductivity Density Trends Graphs Plots Discussed Explained Ks5 Revision Notes

Periodic Trends Variation In Atomic Radii Of Elements In Different Blocks Chemistry Stack Exchange
Periodicity Trends Along Period 3 A Level Chemistry Study Mind